.jpg?width=1600&height=900&name=Medical%20Writing%20for%20Medical%20Devices%20-%20Clinical%20-%20QbD%20Group%20(1).jpg)
Experts in Medical Device Clinical Trials
QbD Clinical is a European expert Clinical Research Organization (CRO), specializing in clinical trials for medical devices. We partner with companies worldwide, ensuring they meet the highest quality and safety standards, from concept to patient. We bring passion to everything we do, whether it’s a brief consultation or a full-service clinical trial.
How can we help?
We have in-depth expertise in the challenges associated with medical devices, with a focus on cardiology, vascular, neurology, and orthopedics. Unlike CROs that primarily work with pharmaceuticals, we specialize exclusively in medical device clinical trials, offering expert support tailored to your needs.
Neurology
- Alzheimer's disease
- Parkinson's disease
- Multiple sclerosis
- Epilepsy
- Stroke
- Others
Orthopedic
- Osteoarthritis
- Rheumatoid arthritis
- Spinal disorders
- Fracture repair
- Others

Why QbD Group?
With over 10 years of experience, QbD Clinical delivers flexible, tailored clinical solutions to help bring medical devices to market efficiently while ensuring compliance with MDR and ISO 14155.
- +52 therapeutic indications
- +250 clients worldwide
- +50 countries covered
- +92 clinical experts worldwide
- +200 clinical trials
- +650 projects delivered
- +2400 clinical sites
Always glad to share our expertise
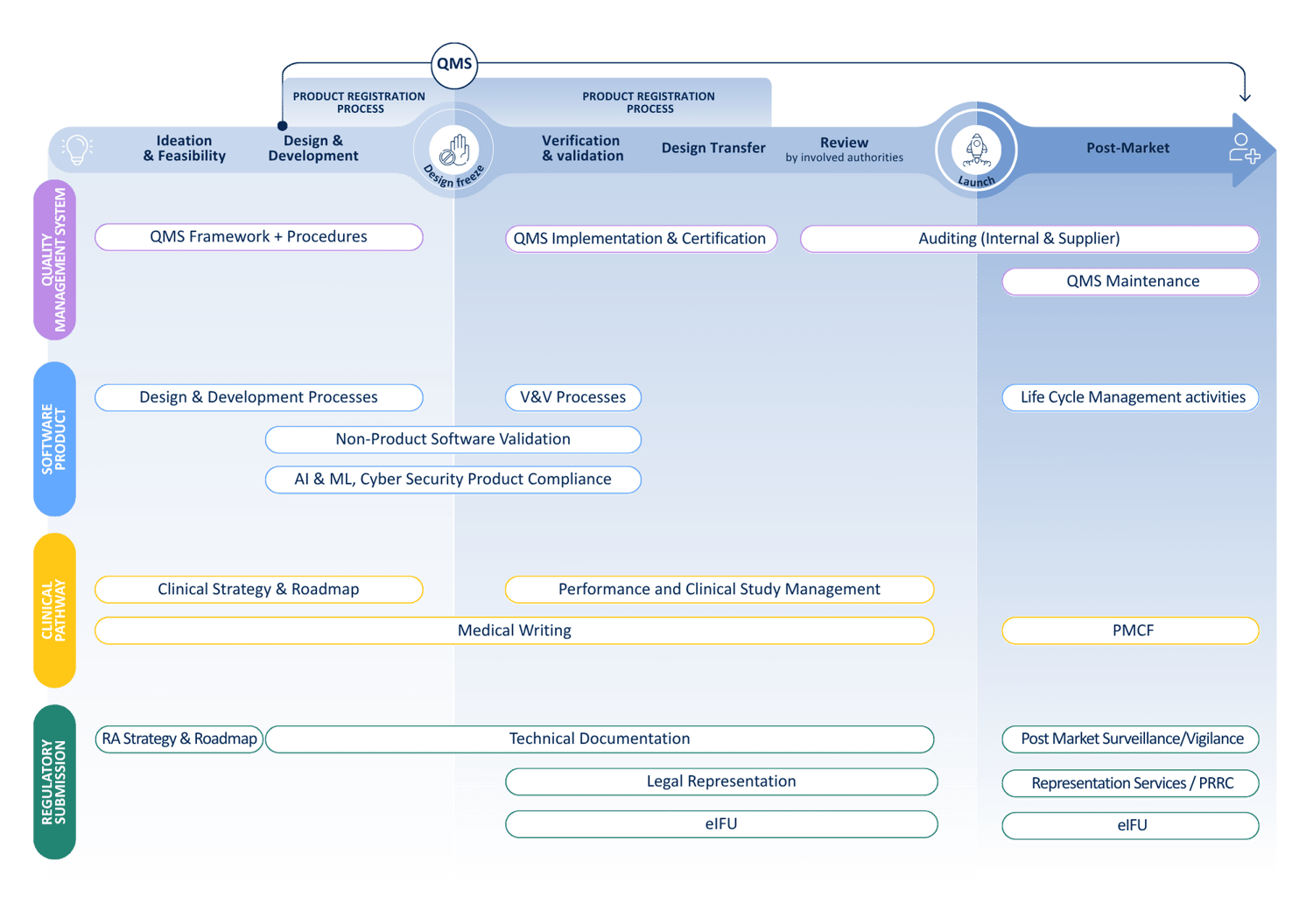
Your journey from idea to patient
Meet our experts
Conducting clinical trials for medical devices requires a unique combination of regulatory insight, clinical expertise, and a deep understanding of device-specific requirements. At QbD Group, our experts are committed to designing and managing high-quality, cost-efficient clinical investigations that meet both regulatory expectations and your business goals.
Discover the dedicated professionals who guide your medical device trials from concept to clinical success.
Kristof Vanschoonbeek
- Dual Leadership Roles at QbD Clinical
- Expertise in PMCF Survey Management
- Team Leadership and Development
- Extensive Background in Clinical Research and Academia
Julie Hendrickx
- International experience in Pharma and MD field
- 8+ years as Clinical Project Manager
- Project Management from study start-up to close-out
Petra De Geest
- MD clinical evidence and medical writing (Class I to Class III, including MDSW and AI-driven MDSW)
- Safety Management in MD clinical investigations
- Strategic leadership & consultancy
Sarah Andries
- 10 years in Clinical Regulatory
- Regulatory Authority & Ethics committee submissions
- MDR & National legislations and requirements (EU)
- EU Legal Representative
Recent client cases: real-world results across therapeutic areas
CRO services
In addition to operational support, our experts offer services in medical and technical writing, quality and safety management, clinical regulatory affairs, and data management.
Feasibility
Regulatory submissions
Medical writing
Data management
Monitoring
Site selection and management
Project management
Safety management (CEC, DSMB)
Supply management
Core lab management
Quality
Legal representative
Resources











.jpg)










.png?width=109&height=108&name=Pharma%20(2).png)
.png?width=111&height=108&name=Medical%20Devices%20(2).png)
.png?width=84&height=107&name=IVD%20(2).png)

.png?width=652&height=652&name=Julie%20Hendrickx%20(1).png)