Medical Devices
Regulatory expertise you can trust
Comprehensive quality and clinical solutions
Post-market monitoring and maintenance
Offering comprehensive Medical Device services
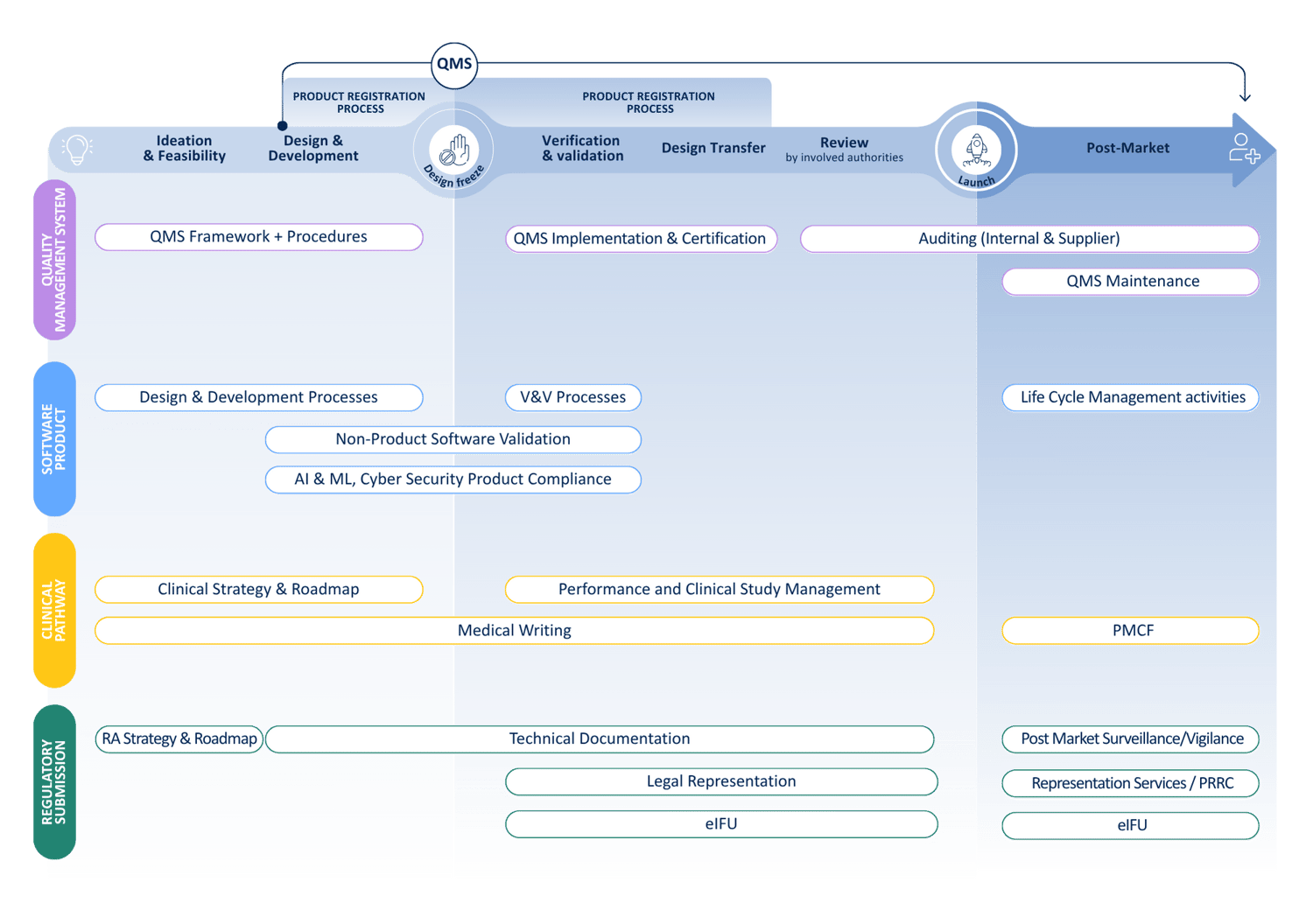
We provide end-to-end support across the entire MD lifecycle. Our services include:
.jpg?width=1600&height=900&name=Legal%20Representative%20-%20Regulatory%20Affairs%20-%20QbD%20Group%20(4).jpg)
Authorized & legal representation
Navigating regulatory approvals in the EU? We act as your trusted legal representative, ensuring compliance with stringent European standards.
- Strategic regulatory guidance
- Submission planning & execution
- Ethics committee & competent authority submissions
- Study modifications & amendments

Digital solutions
We offer cutting-edge software solutions to streamline regulatory compliance and quality management.
- Scilife (Smart QMS): Digital QMS for inspection readiness, risk mitigation, and compliance tracking.
- IFUcare: A full-service eIFU solution for digital technical documentation distribution.
.jpg?width=1600&height=900&name=Companion%20Diagnostics%20Services%20-%20QbD%20Group%20(2).jpg)
Outsourcing solutions
Need extra expertise for your medical device projects? We provide flexible outsourcing solutions to support your team with:
- Regulatory Affairs & Quality Assurance Specialists:
- Clinical Research & Performance Evaluation Experts
-
Post Market Surveillance (PMS) &
Post-Market Clinical Follow-up (PMCF) -
Project Management & Medical Writing Support
We cover the full Medical Device life cycle
From regulatory expertise to quality assurance and clinical evidence, we partner with you to accelerate your journey from idea to patient.
Meet our experts
Bringing a medical device to market demands specialized expertise in regulatory strategy, clinical evaluation, quality management, and compliance with evolving standards. At QbD Group, our experts blend in-depth industry knowledge with practical experience to guide you seamlessly through every phase of development, approval, and post-market surveillance.
Get to know some of our key team members who are dedicated to supporting your medical device journey.
Kristof Vanschoonbeek
20+ years of experience in clinical research including 3 years in the MD field.
- Dual Leadership Roles at QbD Clinical
- Expertise in PMCF Survey Management
- Team Leadership and Development
- Extensive Background in Clinical Research and Academia
Julie Hendrickx
20+ years clinical project delivery experience.
- International experience in Pharma and MD field
- 8+ years as Clinical Project Manager
- Project Management from study start-up to close-out
Petra De Geest
12 years experience in MD field.
- MD clinical evidence and medical writing (Class I to Class III, including MDSW and AI-driven MDSW)
- Safety Management in MD clinical investigations
- Strategic leadership & consultancy
Sarah Andries
12 years of experience in MD field.
- 10 years in Clinical Regulatory
- Regulatory Authority & Ethics committee submissions
- MDR & National legislations and requirements (EU)
- EU Legal Representative
Anne-Sophie Grell
20+ years experience in QA & RA for Medical Devices.
- Extensive experience in Regulatory Compliance and Quality Assurance
- Proven leadership in Medical Device Regulatory Affairs
- In-depth understanding of industry standards and global regulations
- Recognized authority in ensuring compliance and driving successful outcomes
Caroline Aernouts
- Expertise in Medical Device Software (MDSW)
- 5 years of prior experience at Materialise focused on MDSW and custom-made implants
- Strong background in navigating regulatory requirements for innovative medical technologies
Pieter Smits
Extensive experience in EU and US regulations and standards for SaMD/MDSW
- Specialized knowledge in AI and cybersecurity regulatory requirements
- Expertise in MDR/IVDR and FDA compliance, ISO 13485, IEC 62304, IEC 82304, ISO 62366, ISO 81001, and ISO 14971
- Proven project management experience in the pharmaceutical and medical device industries
- Dedicated to bridging compliance and innovation in the evolving SaMD/MDSW landscape
How we can help you
QbD Group offers a comprehensive suite of services tailored to address the unique needs of medical device manufacturers.
Why QbD Group?
YOUR MEDICAL DEVICES INDUSTRY EXPERT
From full-service clinical studies to technical documentation, our experts support you through every stage of the MDR certification process to ensure your device reaches the market safely and compliantly.
Trusted by Notified Bodies
Globally recognized MDR experts
10+ years of experience
On top of industry trends

Get in touch
Resources




Get the latest industry news
Staying on top of the latest in the life science industry can be a daunting task. This newsletter will keep you up-to-date with the latest news, blogs and webinars so you can keep ahead of the curve.
Subscribe on LinkedIn










.jpg?width=1600&height=900&name=Medical%20Writing%20for%20Medical%20Devices%20-%20Clinical%20-%20QbD%20Group%20(1).jpg)
.jpg?width=1600&height=900&name=Expert%20Regulatory%20%26%20Quality%20Support%20for%20MedTech%20Start-ups%20(1).jpg)


.png?width=1600&height=900&name=MDR%20Compliance%20%E2%80%93%20CE%20Certification%20for%20Medical%20Devices%20%20(2).png)
.png?width=720&height=720&name=Kristof%20Vanschoonbeek%20(NO%20BG).png)
.png?width=652&height=652&name=Julie%20Hendrickx%20(1).png)





