
Combination Products
Navigating regulatory pathways can often feel like a maze, leading to uncertainty and potential pitfalls in the submission process. This is even more so when it comes to combination products.
Nothing is more disheartening than investing significant time and resources into developing your combination product, only to face rejection from regulatory authorities due to unclear expectations or insufficient data.
At Qbd Group, we offer comprehensive solutions to navigate the complex process of registering these innovative products in both the European Union (EU) and the United States (US).
Device-led Combination Products
If your product is classified as a medical device with an ancillary medicinal substance, it must comply with EU MDR 2017/745.
- The Notified Body must consult the EMA for a scientific opinion on the excipient if:
- It is derived from human blood or plasma.
- It has been previously assessed by the EMA.
- It falls under the centralized procedure.
In other cases, the Notified Body may consult either:
-
The National Competent Authority, or
-
The EMA (e.g. if the same substance has been evaluated before).
Medicinal Product-led Combination Products
If your product is primarily a medicinal product, the combination is regulated under the Medicinal Product Directive (2001/83/EC).
The type of product — and how the medical device is integrated — influences the required documentation and regulatory steps.
Types of medicinal combination products:
-
Integrated: The device is part of the product (e.g. prefilled syringe).
-
Co-packaged: Medicinal product and device are packaged together (e.g. inhaler + cartridge).
-
Referenced: The product refers to a separate device used during administration.
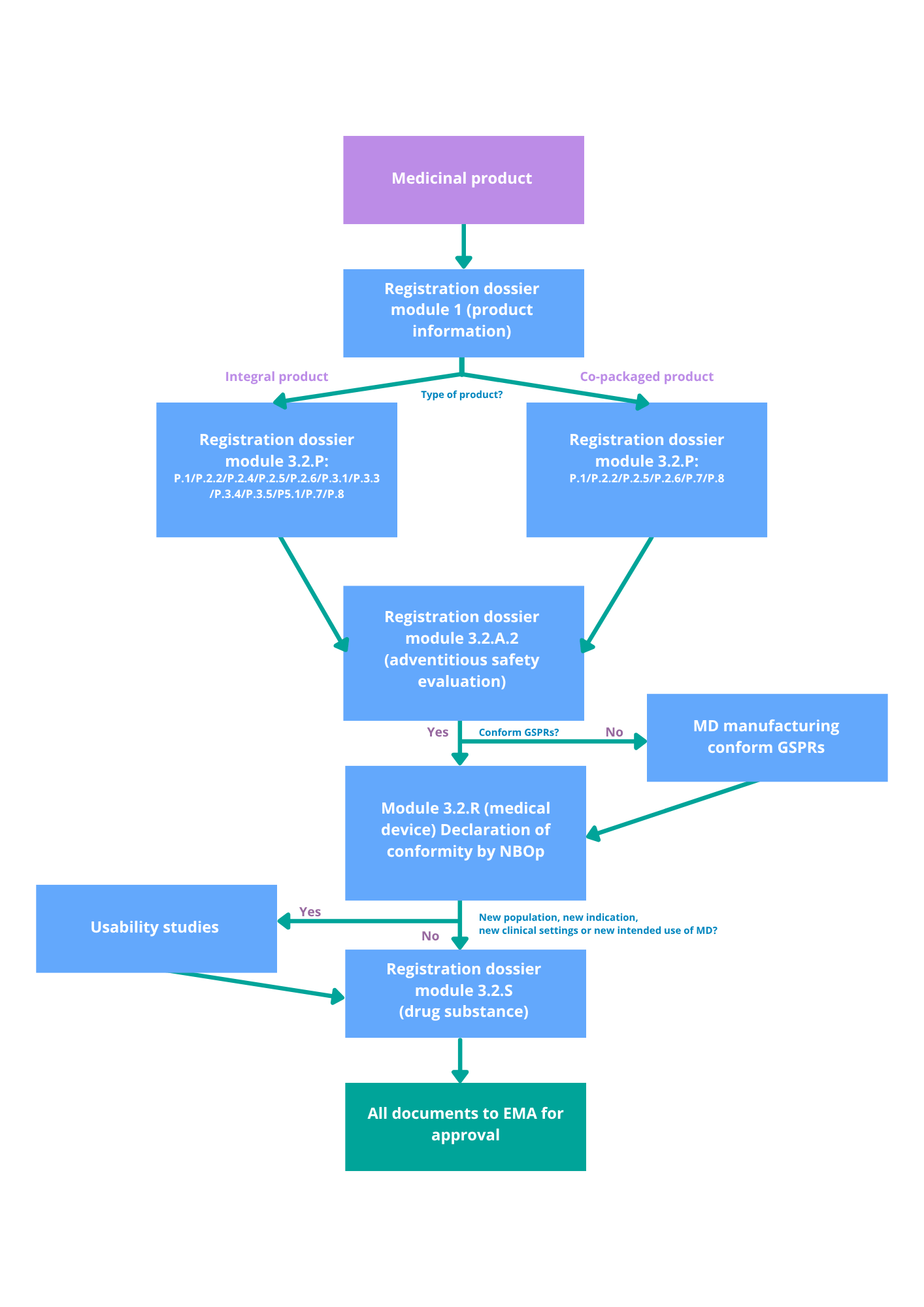
Your regulatory roadmap: key steps
Use the flowchart below as a guide to determine:
-
Which authority is involved.
-
What quality documentation is needed.
-
How to prepare your submission for compliance.
Examples of Combination Products
|
Example |
Primary Mode | Pathway |
|---|---|---|
| Drug-eluting stent | Device | MDR + EMA opinion |
| Prefilled syringe | Drug | MPD |
| Contraceptive patch | Drug | MPD |
| Coated catheter | Device | MDR |
Regulatory pathway in the USA

Step 1: identify the lead FDA center
-
Depends on the PMOA (drug, device, or biologic).
-
If unclear, you may submit a Request for Designation (RFD) to the FDA.
-1.jpg?width=540&height=300&name=External%20newsletters%20rechthoek%20(2)-1.jpg)
Step 2: determine the type of investigational application
Depending on the PMOA, one of the following may apply:
-
IND (Investigational New Drug Application) → Used when the primary mode is drug or biologic.
-
IDE (Investigational Device Exemption) → Used when the primary mode is device.
Take into account the combination product as a whole, not just its individual components.

Step 3: select the appropriate marketing application
The marketing application depends on the constituent part with the PMOA:
| PMOA | Marketing Application |
|---|---|
| Drug | NDA (New Drug Application) or ANDA (Abbreviated NDA) |
| Biologic | BLA (Biologics License Application) |
| Device | PMA (Premarket Approval), De Novo, or 510(k) Notification |
Why choose us for combination product lifecycle management?
Expert guidance on procedures
End-to-end lifecycle management
In-depth regulatory knowledge
Why QbD Group?
At QbD Group, we provide deep expertise to guide life science companies through complex regulatory landscapes and ensure compliance throughout the product lifecycle.
Our team understands the stringent requirements needed to safely and effectively develop, approve, and distribute combination products.
Beyond market entry, we continue supporting businesses by managing critical processes that ensure the ongoing safety, efficacy, and compliance of their products before and after they reach patients.
+10 years of experience
Full lifecycle support
Global presence
Best managed company

Get in touch
Have questions about the regulatory pathways for your combination product?
We understand the complexities of navigating EU and US regulations. Whether you’re seeking clarity on the Primary Mode of Action (PMOA), determining the right regulatory pathway, or managing lifecycle requirements, our experts are here to help.
Related expert content
-1.jpg)
.jpg)
.jpg)







%20Checklist.jpg)











.jpg)







.jpg)


Ensuring a smooth MDR transition for Oystershell's medical devices

.jpg)
.jpg)

.jpg)

Get the latest industry news

Connect with us at these events
April
May
June
September










.png)